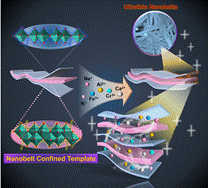
Aqueous zinc-ion batteries (AZIBs) are promising energy storage systems. Unfortunately, the electrostatic repulsion of Zn2+ is significant, and Zn2+ is highly hydrated in aqueous electrolytes. The motion resistance of hydrated Zn2+ in the interlayer space is considerable, resulting in sluggish electrochemical kinetics. The larger radius of hydrated ions (4.3 Å) requires larger diffusion channels, which are fundamental bottlenecks to further promote the practical application of AZIBs. Among the numerous cathode materials, vanadium oxides have received extensive attention due to the open-frame crystal structure that provides a convenient mobility channel for Zn2+.However, certain problems cannot be ignored, such as unstable layered structure and low electronic conductivity, which lead to poor cycle capacity and stability. Among them, introducing metal ions into the interlayer spacing of vanadium-based materials is a common strategy to increase interlayer spacing, expand diffusion channels, and improve ionic conductivity. The interlayer metal-confinement strategy makes it possible to confine metal ions in a specific interlayer spacing without changing the morphology of vanadium oxides.
Recently, Prof. Huan Pang of Yangzhou University and Prof. Yizhou Zhang of Nanjing University of Information Engineering have published a paper entitled "Co-intercalation of Dual Charge Carriers in Metal-ion-confining Layered Vanadium Oxide Nanobelts for Aqueous Zinc-Ion Batteries" in the journal Angewandte Chemie International Edition (Angew. Chem. Int. Ed., DOI: 10.1002/anie. 202216089). This work obtain a series of nanomaterials (Mx-V6O13, M= Na, K, Ag, Ca, Mn, Co, Ni, Cu, Zn, Fe, Cr, Al, etc.) based on metal-confined nanobelts, and describe the effect of interlayer spacing on the electrochemical performance.
The electrochemical properties of the obtained Al2.65V6O13·2.07H2O as cathodes for AZIBs are remarkably improved with a high initial capacity of 571.7 mAh·g−1 at 1.0 A·g−1. Even at a high current density of 5.0 A·g−1, the initial capacity can still reach 205.7 mAh·g−1, with a high capacity retention of 89.2% after 2000 cycles. This study demonstrates that nanobelts confined with metal ions can significantly improve energy storage applications, revealing new avenues for enhancing the electrochemical performance of AZIBs.
Author Team: Tingting Lv, Guoyin Zhu, Shengyang Dong, Qingquan Kong, Yi Peng, Shu Jiang, Guangxun Zhang, Zilin Yang, Shengyang Yang, Xiaochen Dong, Huan Pang*, Yizhou Zhang*.
Original Link.:https://doi.org/10.1002/anie.202216089

